Bob's Research Page
Research Groups
Current Group Members
- Phillip (Aron) McCart, Graduate Student
- Scott Maasen, Graduate Student
- Manelich Luna, Graduate Student
- Lukmon Aminu, Graduate Student
- Adam Brandt, Undergraduate Student
- Sean Anderson, Undergraduate Student
- Dayton Kizzire, Undergraduate Student
- Steve Harrellson, Undergraduate Student
Recent Group Members
- Hao Yan, Postdoctoral Research Associate; Present Position: Scientist, HPstar, Shanghai, China.
- Joe Demster, MS Graduate Student; Present Position: Associate Applications Engineer, Brewer Science, Springfield, MO.
- H.A. Naveen Dharmagunawardhane, MS Graduate Student; Present Position: PhD Student, Stony Brook University, Stony Brook, NY.
- David Lamb, MS Graduate Student; Present Position: Project Engineer, U.S. Department of the Navy, Norfolk, VA.
- Yarden Bosch, Undergraduate Student; Present Position: MS Student, Missouri State University.
- Junho Chung, Undergraduate Student; Present Position: PhD Student, Korea University, Seoul, South Korea.
- Laurel Farris, Undergraduate Student, BS, 2013.
News and Announcements
The Department of Physics, Astronomy and Materials Science at Missouri State University has an opening for a postdoctoral research associate position. As a participant in the Center for Energy Frontier Research in Extreme Environments (EFree), the selected candidate will collaborate with Prof. Robert Mayanovic and other scientists in the center to investigate the physical and chemical properties of primarily nanophase materials in extreme environments. For required qualifications and application procedures, visit https://jobs.missourestate.edu/ or contact RobertMayanovic@missouristate.edu.
Research
The following is presented in two formats:
- The first is for the general audience.
- The latter is more detailed for a scientific audience.
Participation in EFree: Energy Frontier Research in Extreme Environments
EFree is a nation-wide DOE-funded center, directed by Ho-kwang (Dave) Mao of the Geophysical Laboratory of the Carnegie Institution of Washington, involved in fundamental research on materials in extreme environments that is vital to addressing twenty-first century energy needs. EFree is one of 46 Energy Frontier Research Centers funded by the Department of Energy (DOE), a program that was initiated in 2009. MSU is one of 8 partner universities, along with 3 national laboratories and the Geophysical Laboratory that comprise EFree. For further information, please visit https://efree.gl.ciw.edu/content/about-efree.



Research Areas
The main line of research in my laboratory involves the use of synchrotron techniques, Raman spectroscopy, photoluminescence spectroscopy and other characterization methods to investigate the physico-chemical properties of energy-related (nanophase and bulk) materials under extremes of pressure, temperature, radiation, and hydrothermal environments. A very useful instrument of choice in this line of research is the diamond anvil cell. In the diamond anvil cell, two opposing diamond anvils are driven against each other and a metal gasket sandwiched between them (with a hole in the middle that serves as a sample chamber) to make a seal and exert pressure on the sample.
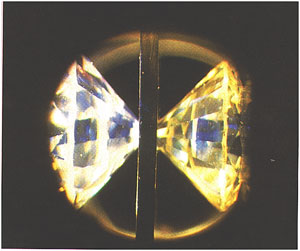
Two opposing diamonds are squeezed against a metal gasket having a hole in the middle in the diamond anvil cell. The volume defined by the small (culet) faces of the diamonds and the hole of the gasket serves as the sample chamber. By squeezing on the diamonds in a press, very great pressures on the sample are attainable.
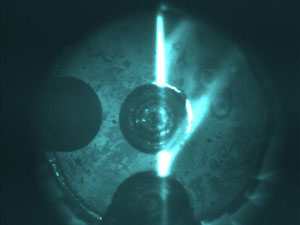
Visible fluorescence (vertical streak) showing the passage of incident x-rays through the sample chamber in the grooved upper diamond anvil of the diamond anvil cell. The streaks to the right are due to the diffraction from the diamond. The two (circular) milled grooves in the anvil allow for passage of lower-energy x-ray photons.
Precipitation of Metal Ions on Nanoparticles in Aqueous Fluids to Supercritical Conditions
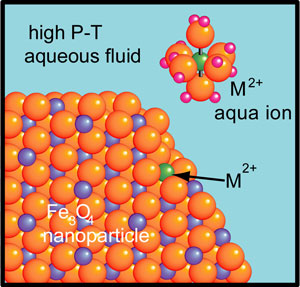
Investigations of the reactivity, adsorption, precipitation, structure, vibrational properties (i.e. Raman spectroscopy), photoluminescence and other properties of metal (e.g., Co, Ni, Zn, Rh, Eu) ions on metal oxide (e.g., Fe3O4, TiO2, Cr2O3) nanoparticles (NPs) in aqueous fluids heated to 500 °C and at pressures up to approximately 500 MPa are made in our laboratory and at the Advance Photon Source (APS), Argonne National Laboratory.
Applications include the operation of supercritical water cooled reactors (SCWRs). For increased efficiency, next-generation power reactors are projected to operate with supercritical water. The resulting severity of oxidation, corrosion and spalling of materials in primary cooling loops requires materials with exceptional structural stability. Spalled ferric nanoparticles, transported with other corrosion byproducts (e.g., Fe3+, Ni2+, Co2+) are a critical failure mechanism via erosion damage and enhanced corrosion that remains poorly understood. Additional potential applications include general catalysis, photocatalysis, photovoltaics, optoelectronics, optical devices, and others. Several metal oxides (e.g., TiO2), especially in nanophase form, show promise for use as photocatalysts, for applications such as conversion of sunlight into renewable energy and for decomposition of organic waste.
There are two general issues that prevent widespread use of present day nanomaterials for photocatalysis: 1) Low catalytic efficiency, and 2) light-absorption in the near UV region of the solar spectrum.
New materials must be designed to overcome these issues before their use is made practical in photocatalytic cells. The data, obtained at the atomic and nano scale from these systems, will aid in the future design of materials.
Investigations of Mesoporous Materials under Extreme Conditions
Periodic mesoporous materials have tiny pores (2 - 50 x 10-9 m in size) and typically consist of SiO2, Al2O3 and SiO2, or a metal oxide. Mesoporous materials are useful in the chemical industry and have potential for use in energy conversion. Our interest is in the study of the properties of mesoporous materials under the extremes of pressure and temperature and in high pressure-temperature aqueous fluids.
Due to their large surface area and pore structure, periodic mesoporous materials are decidedly suitable for chemical and energy applications (e.g., heterogeneous catalysis and ion-exchange). In order to have direct applicability for energy conversion under extreme conditions, it is important that periodic mesoporous materials have high thermal and hydrothermal stability. Potential applications of synthetic periodic mesoporous materials include supercritical water gasification of biomass and in hydro- or steam-cracking of petroleum. Our research involves use of synchrotron techniques, micro-Raman spectroscopy, and photoluminescence spectroscopy for studies of the hydrothermal and mechanical stability of periodic mesoporous materials (silica, carbon, alumino-silica, etc.).
Structural Properties of Hydrous Silicate Glasses and Melts
Silicate (SiO2 based) glasses lack the periodic structure of crystalline materials. We aim to study the structural behavior of silicate glasses and melts that have water incorporated within their structures. Through our studies, it is anticipated that these systems will be better understood and that we will be able to predict and synthesize new glassy materials specifically for energy applications under extreme conditions.
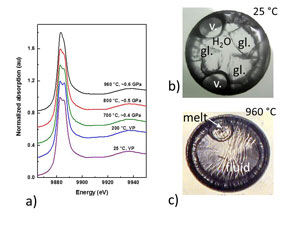
(b) an image of the glass+H2O sample at 25 °C (at vapor pressure), and
(c) an image of the melt+silicate-rich aqueous fluid sample at 960 °C (at ~0.6 GPa).
The questions that need to be addressed are:
1) how soluble are the oxide and silicate-based glasses and what are the transport properties of the critical dissolved
constituent elements in high P-T aqueous fluids, and,
2) what are the structure properties of the glass-fluid system under extreme P-T conditions?
We have recently completed an in situ Ta L3-edge XAS study of a hydrous Ta-bearing aluminosilicate glass/melt and a silicate-bearing aqueous fluid to 960 °C and approximately 0.6 GPa. The Ta L3-edge XANES in the region of the white-line doublet vary with increasing P-T conditions of the silicate glass/melt+water system, indicating a shift in the electronic density of states in the vicinity of quasi bound Ta 5d states probed by the 2p3/2 core photoelectron. Ab initio modeling of the Ta L3-edge XANES shows that local structure surrounding the 6-fold coordinated Ta in the hydrous silicate glass/melt becomes progressively more distorted with increasing P-T conditions.
Similarly, our results show that the structure of Ta-Qn complexes in the silicate bearing aqueous fluid is distorted in a seemingly continuous fashion with respect to that of the melt. The angular-momentum projected density of states (l-DOS) calculations show that the upper doublet level quasi-bound d-DOS is progressively reduced with increasing distortion of the Ta-Qn cluster in the system.
Additional Characterization Tools
Raman-PL Spectroscopy
A LabRAM HR800 micro-Raman instrument is located in our laboratory. The LabRAM HR800 has confocal Raman and photoluminescence spectroscopic capability and has a detection range from 200 to ~4000 nm. The instrument includes:
- a stigmatic 800 mm spectrometer equipped with four gratings (150, 300, 600, and 2400 gr/mm),
- an Andor-Newton electron-multiplying 1600x200 pixel CCD detector, and
- a Ln-cooled Symphony II InGaAs array detector.
The LabRAM HR800 has 3 laser excitation sources: a 50 mW 532 nm narrow bandwidth laser, a 80 mW 785 nm diode laser, and a 30 mW 325 nm HeCd laser, filter sets for Stokes Raman excitation, and it has a 2-grating spectrograph with resolution to 0.3 cm-1. The system has duo-scan and motorized mapping capability and was obtained from an NSF-DMR grant (1126375).

The Horiba LabRAM HR800 micro-Raman/photoluminescence spectroscopy instrument.

The Bruker Apex diffractometer (Laue type) housed in the X-Ray Analysis Facility lab (KEM 203).
In addition, the department has available an x-ray analysis facility, housing a powder/thin-film diffractometer and a single crystal Laue diffractometer (Bruker), a Quantum Design SQUID magnetometer capable of magnetization measurements from 5 to 300 K and to 5 T, a field emission scanning electron microscope (FE-SEM) FEI Quanta 200 FEG having EDX mode capability, a pulsed-laser thin film deposition system, and other characterization tools.
Select Publications
H. Yan, R.A. Mayanovic, J. Demster and Alan J. Anderson. In situ monitoring of the adsorption of Co2+ on the surface of Fe3O4 nanoparticles in high-temperature aqueous fluids, J. Supercrit. Fluids http://dx.doi.org/doi: 10.1016/j.supflu.2013.05.017 (2013).
R.A. Mayanovic, H. Yan, A.J. Anderson, G. Solferino, Investigation of the structural environment of Ta in a silicate glass and water system under high P-T conditions, J. Non-Crystal. Solids 368, 71-78 (2013).
R.A. Mayanovic, H. Yan, A.J. Anderson, P.R. Meredith, W.A. Bassett, In situ x-ray absorption spectroscopic study of the adsorption of Ni2+ on Fe3O4 nanoparticles in supercritical aqueous fluids. J. Phys. Chem. C 116, 2218-2225 (2012).
H. Yan, R.A. Mayanovic, J. Demster and Alan J. Anderson (2012). In situ XANES Study of Co2+ Ion Adsorption on Fe3O4 Nanoparticles in Supercritical Aqueous Fluids. MRS Proceedings, 1383, mrsf11-1383-a07-10 doi:10.1557/opl.2012.183
H. Yan, R.A. Mayanovic, A.J. Anderson, P.R. Meredith, An in situ x-ray spectroscopic study of Mo6+ speciation in supercritical aqueous solutions. Nucl. Instr. Methods Phys. Res. A, 649, 207 (2011).
R.A. Mayanovic, A.J. Anderson, W.A. Bassett, and I.-M. Chou, Steric hindrance and the enhanced stability of light rare-earth elements in hydrothermal fluids, Am. Mineral. 94, 1487-1490 (2009).
R.A. Mayanovic, A.J. Anderson, W.A. Bassett, and I.-M. Chou, Spectroscopic studies of Eu/HNO3 aqueous solutions at high temperatures and pressures and Nb-bearing silicate melt phases coexisting with hydrothermal fluids by improved mounting of a modified hydrothermal diamond anvil cell. Rev. Sci. Instr. 78, 053904:1-9 (2007).
W.A. Bassett, I.-M. Chou, A.J. Anderson, and R.A. Mayanovic, Aqueous chemistry in the diamond anvil cell up to and beyond the critical point of water, in Chemistry at Extreme Conditions, M. R. Manaa, ed. (Elsevier, 2005) pp 223-240.
R.A. Mayanovic, A.J. Anderson, W.A. Bassett, and I.-M. Chou, Hydrogen Bond Breaking in Aqueous Solutions near the Critical Point. Chem. Phys. Lett. 336, 212 (2001).
Links to Additional Resources
The Advanced Photon Source (APS) synchrotron facility at Argonne National Laboratory:
http://www.aps.anl.gov/
The Cornell High Energy Synchrotron Source (CHESS) in Ithaca, NY:
http://www.chess.cornell.edu/
The Department of Physics, Astronomy & Materials Science at Missouri State University:
https://physics.missouristate.edu/