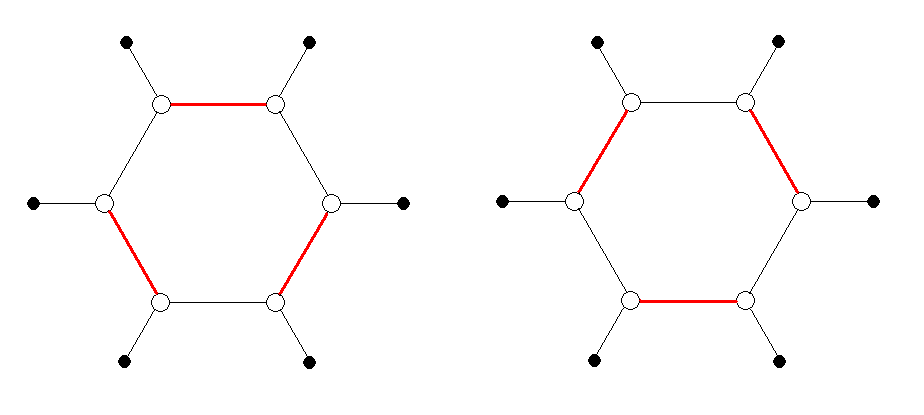

In organic chemistry, a carbon atom has four bonds that can be used to attach it to adjacent atoms. The connection to an adjacent atom can be a single, double, or triple bond. The 19th century view of benzene (C6H6) was that it oscillates between two "resonance structures" each consisting of alternating double and single bonds (so that each carbon atom has four bond emanating from it). This is illustrated in the figure below. Open circles represent carbon atoms, filled circles represent hydrogen atoms, black lines are single bonds, and red lines are double bonds.

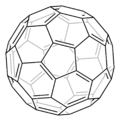
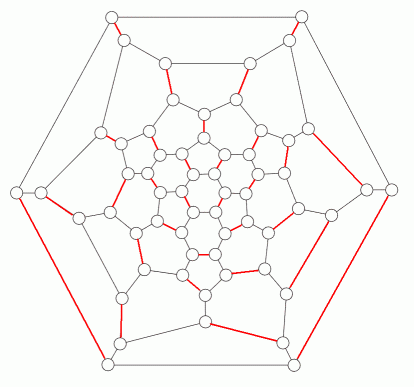
In 1985, buckminsterfullerene (C60) was discovered. Its carbon atoms lie at the vertices of a truncated icosahedron. One of its resonance structures is shown below (in perspective, with single bonds as one line and double bonds as two). A second resonance structure (from a graph theoretical point of view, using the same conventions as the representations of benzene above) is given below that.
This months's problem is to determine the total number of resonance structures of buckminsterfullerene.